Lakewood Ranch Medical Center Performs its First Barostim Heart Failure Treatment

Vascular surgeon Vivian M. Torres, MD, successfully implanted the first Food and Drug Administration (FDA)-approved heart failure device, Barostim™ Baroreflex Activation Therapy, in a patient of interventional cardiologist Vivek Kumar, DO, at Lakewood Ranch Medical Center on Monday, March 7, 2022.
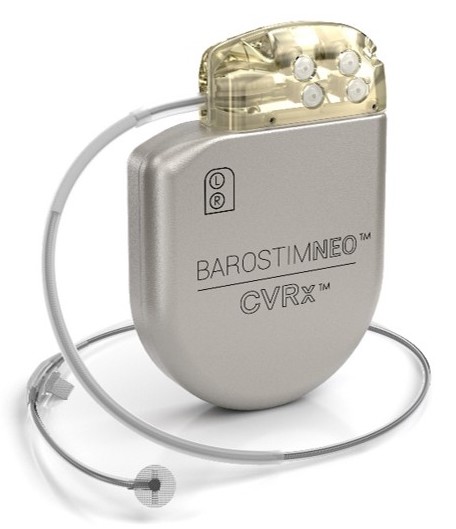 Barostim is designed to address medical needs in heart failure patients when medication has not been effective in reducing symptoms and improving quality of life. Barostim is a simple, implantable device similar to a pacemaker. It electrically activates the body’s main cardiovascular reflex, the baroreflex, signaling the brain to regulate cardiovascular function resulting in lower heart rate and blood pressure, helping to relieve the symptoms of heart failure. The device targets the nerves around the heart and not the heart itself.
Barostim is designed to address medical needs in heart failure patients when medication has not been effective in reducing symptoms and improving quality of life. Barostim is a simple, implantable device similar to a pacemaker. It electrically activates the body’s main cardiovascular reflex, the baroreflex, signaling the brain to regulate cardiovascular function resulting in lower heart rate and blood pressure, helping to relieve the symptoms of heart failure. The device targets the nerves around the heart and not the heart itself.
“I am grateful to bring this innovative technology and treatment option to Lakewood Ranch Medical Center,” says Dr. Torres. “This procedure helps improve a heart failure patient’s quality of life and lessen discomfort in certain patients who have symptoms despite other therapies.”
Advanced Cardiovascular Services
The procedure is performed under general anesthesia and is an outpatient procedure. Patients are usually discharged the same day.
Candidates for this procedure are patients who have symptoms of heart failure including shortness of breath, fatigue and decreased tolerance for exercise, and have met other criteria.
